Potassium Hydroxide And Iron Ii Nitrate Precipitate
32 e Aluminium also bears minor similarities to the metalloid boron in the same group. Solubility of iron and iron compounds.

Chapter 8 Reactions In Aqueous Solutions Ppt Download
B What is the limiting reactant in the reaction.

. A 427 gram sample of potassium nitrate contains how many grams of potassium. A common example is table salt with positively charged sodium ions and negatively charged chloride ions. Beryllium is a chemical element with the symbol Be and atomic number 4.
Solution a The two possible products for this combination are KNO 3 and BaSO 4. A potassium sulfate and barium nitrate b lithium chloride and silver acetate c lead nitrate and ammonium carbonate. A solution of lead II nitrate is dropped into a solution of potassium iodide forming a brilliant yellow lead II iodide precipitate.
C When solid sodium chloride is added to aqueous sulfuric acid hydrogen chloride gas and aqueous. The component ions in a salt compound can be either inorganic such as. Gunpowder has been widely used as a propellant in firearms artillery rocketry.
Discovered by French chemist Paul-Émile Lecoq de Boisbaudran in 1875 Gallium is in group 13 of the periodic table and is similar to the other metals of the group aluminium indium and thalliumGallium exhibits relatively less similarity with boron due to latter being small in atomic size and lacking its reach. To this solution is added 500 mL of 150 M hydrochloric acid and a precipitate forms. To take back the white colour hydrogen peroxide is reacted with lead sulfide.
A Solid potassium chlorate KClO 3 decomposes to form solid potassium chloride and diatomic oxygen gas. Chemical equations are used to graphically illustrate chemical reactions. Zinc hydroxide reacts with phosphoric acid H 3 PO 4 to produce zinc phosphate and water.
Give a reason for the following. 140 g of silver nitrate is dissolved in 125 mL of water. Potassium is a silvery-white metal that is soft enough to be cut with a knife with little force.
Add 2 - 3 drops of the compound in methanol to 2 - 3 mL of. PDF On May 8 2018 Gaurav Kumar Sharma and others published Textbook of Cosmetic Formulations Find read and cite all the research you need on ResearchGate. They are separated by an arrow which indicates the direction and type of the reaction.
Closely related are aluminium oxide hydroxide AlOOH and aluminium oxide or alumina Al. Ferrous ion ironii is oxidized to ferric ion ironiii and H 2 O 2 is reduced to water. Then add dilute ammonium hydroxide dropwise until the precipitate just dissolves.
D Physical state is changed. Then lead sulfate a white precipitate is given. 1 2 1 2 b.
A sample of 705 mg of potassium phosphate is added to 150 mL of 0050 M silver nitrate resulting in the formation of a precipitate. To 1 mL of silver nitrate solution add a few drops of sodium hydroxide. Preparation of the reagent.
2 g of ferrous sulphate crystals are heated in a dry boiling tube. B Refer to answer 15. 2 days ago Potassium hydroxide is an inorganic compound with the formula KOH and is commonly called caustic potash When carbon dioxide CO2 dissolves in rainwater or in the ocean it makes a weak acid Dissolve Aluminum with a Strong Base This step is a single reaction for which the relevant species are H 2 AlOH 4 Al KOH and K The alcoholcatalyst mix is.
In sea regions atmospheric salt particles may play an important role in this process. In chemistry a salt is a chemical compound consisting of an ionic assembly of positively charged cations and negatively charged anions which results in a compound with no net electric charge. It is a steel-gray strong lightweight and brittle alkaline earth metalIt is a divalent element that occurs naturally only in combination with other elements to form minerals.
I CH3OH II O2 III CO2 IV H2O I II III IV a. IronIII chloride B potassium chloride C magnesium chloride D barium chloride. The tip of the arrow points in the direction in which the reaction proceeds.
B Molybdenum is used in the manufacture of ammonia. Notable gemstones high in beryllium include beryl aquamarine emerald and chrysoberylIt is a relatively rare element in the universe. 1 3 1 2 c.
Reacting with potassium hexacyanoferrateII K 4 FeCN 6 solution a Procedure. Gallium is a chemical element with the symbol Ga and atomic number 31. A Write the molecular equation for the reaction.
They consist of chemical or structural formulas of the reactants on the left and those of the products on the right. B Solid aluminum metal reacts with solid diatomic iodine to form solid Al 2 I 6. The HaberBosch process relies on catalysts to accelerate the hydrogenation of N 2The catalysts are heterogeneous meaning that they are solids that interact on gaseous reagentsThe catalyst typically consists of finely divided iron bound to an iron oxide carrier containing promoters possibly including aluminium oxide potassium oxide calcium oxide.
IronIII ion Fe 3 Method I. With ferrous ion in acidic medium Fe 2 H 2 O 2 Fe 3 H 2 O. Many methods are utilized in determining end points of these reactions but the most important method the formation of a coloured precipitate will be considered here.
As such aluminium is the most electropositive metal in its group and its hydroxide is in fact more basic than that of gallium. AlX 3 compounds are valence isoelectronic to BX 3 compounds they have the same valence electronic structure and both behave. Sodium hydroxide reacts with iron III nitrate to create a precipitate of iron III hydroxide in a solution of sodium nitrate.
C Precipitate is formed. Iron II hydroxide often precipitates in natural waters. 2 5 2 2.
Mercury II oxide decomposes to produce mercury and oxygen. The arrow is read as the word yields. A Zinc reacts with silver nitrate to produce zinc nitrate and silver.
B Potassium iodide reacts with lead nitrate to produce potassium nitrate and lead iodide. The solubility guidelines indicate BaSO 4 is insoluble and so a precipitation reaction is expected. I About 2 cm 3 of ironIII chloride solution is poured into a test tube.
Bayerite doyleite and nordstranditeAluminium hydroxide is amphoteric ie it has both basic and acidic properties. The net ionic equation for this reaction. The ironII ion combines with a complex ion in the reagent to produce a dark blue precipitate.
Aluminium hydroxide AlOH 3 is found in nature as the mineral gibbsite also known as hydrargillite and its three much rarer polymorphs. C Calculate the theoretical yield in grams of the precipitate that forms. 2 3 2 4 d.
Fe 2 aq FeCN 6 3-aq dark blue precipitate. Sulfur dioxide and oxygen combine to produce sulfur trioxide. Electrolytes are mostly iron II sulphate which forms during corrosion by atmospheric SO 2.
What is the concentration in units of moiarity of silver ions remaining in the solution. C A blue solution of copper sulphate changes to green when a piece of iron is added to this solution. Elementary iron dissolves in water under normal conditions.
Potassium is the chemical element with the symbol K from Neo-Latin kalium and atomic number 19. Gunpowder also commonly known as black powder to distinguish it from modern smokeless powder is the earliest known chemical explosiveIt consists of a mixture of sulfur carbon in the form of charcoal and potassium nitrate The sulfur and carbon act as fuels while the saltpeter is an oxidizer. Potassium metal reacts rapidly with atmospheric oxygen to form flaky white potassium peroxide in only seconds of exposure.
In the titration of a neutral solution of chloride ions with silver nitrate a small quantity of potassium chromate solution is added to serve as the indicator. A It is a displacement reaction. It was first isolated from potash the ashes of plants.
A 391 B 165 C 214 D 854. Write a balanced equation describing each of the following chemical reactions. A Silver nitrate solution is kept in coloured bottles.
Does Potassium Acetate And Barium Nitrate Form A Precipitate Quora
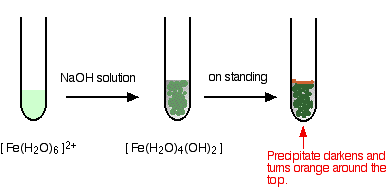
Iron
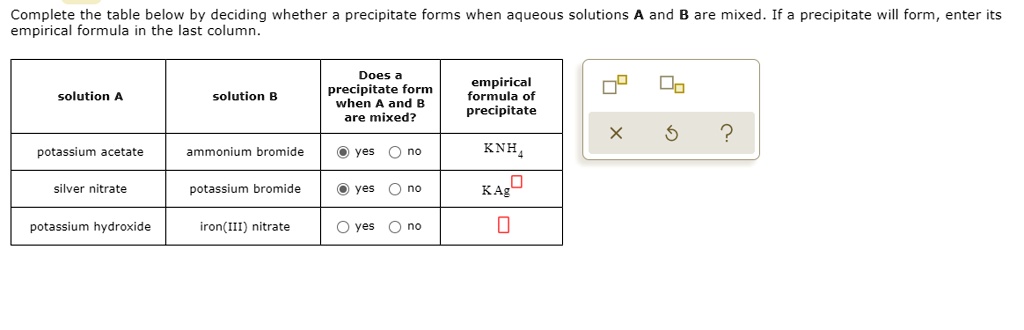
Solved Complete The Table Below By Deciding Whether Pree Cipitate Forms When Aqueous Solutions Empirical Formula In The Last Column And B Are Mixed If A Precipitate Will Form Enter Its Does

Oneclass Lead Ions Can Be Removed From Solution By Precipitation Withsulfate Ions Suppose That A So
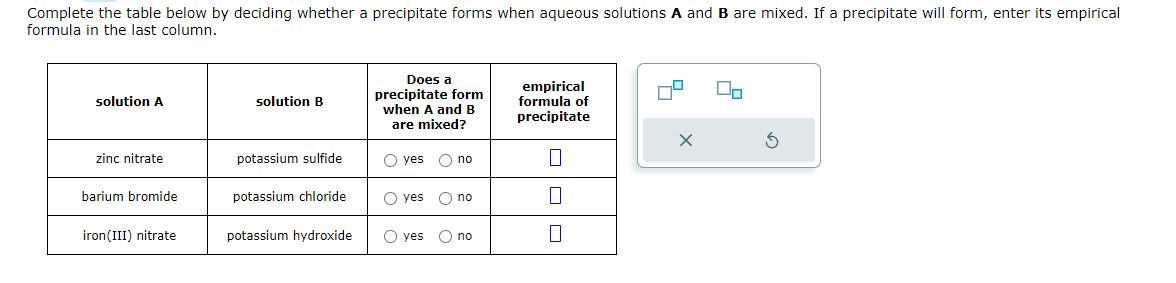
Xx5jpvkwzkyjvm
Chapter 5 Homework Packet
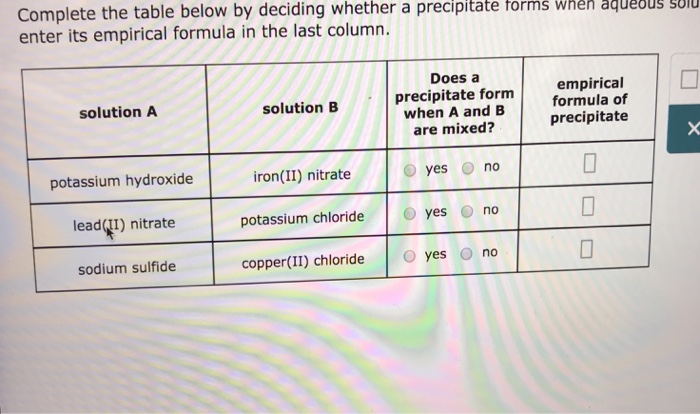
Solved Complete The Table Below By Deciding Whether A Chegg Com

Please Help Which Of The Following Correctly Shows The Net Ionic Equation For The Reaction Between Brainly Com

Solved O Chemical Reactions Predicting Precipitation Chegg Com

4 2 Precipitation Reactions Chemistry Libretexts

When 18 0 Grams Of Iron Iii Nitrate Are Mixed With A Soluti Quizlet
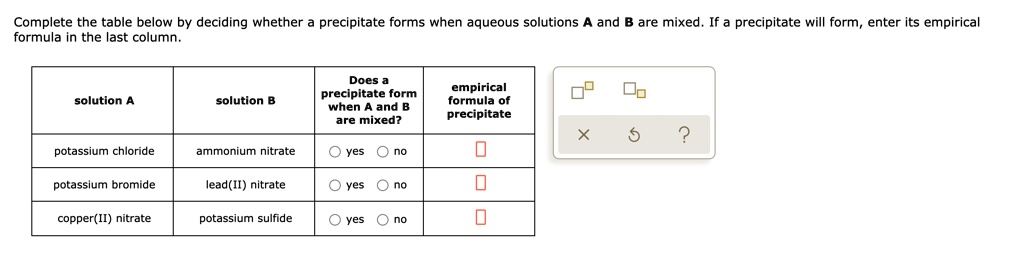
Solved Complete The Table Below By Deciding Whether Precipitate Forms When Aqueous Solutions And Are Mixed If A Precipitate Will Form Enter Its Empirical Formula In The Last Column Does Empirical Formula

Chemistry Confirmantory Test For Iron Ii Iron Iii Lead Ammonium

How To Write The Net Ionic Equation For Fe No3 3 Koh Kno3 Fe Oh 3 Youtube
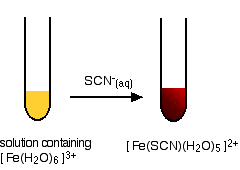
Chemistry Of Iron Chemistry Libretexts

Oxidative Precipitation As A Versatile Method To Obtain Ferromagnetic Fe3o4 Nano And Mesocrystals Adjustable In Morphology And Magnetic Properties Granath 2021 Particle Particle Systems Characterization Wiley Online Library

Potassium Hydroxide Wikipedia